Medical Manufacturing
Medical converting and vast material knowledge paired with vertical integration extends PLITEK®’s ability to provide a sound medical manufacturing solutions. We operate multiple state-of-the-art environmentally-controlled ISO Class 8 clean rooms for manufacturing, assembly, material packaging, components, and finished product.
Innovative medical manufacturing capabilities, proven expertise, consistency, and strict quality standards keep PLITEK® a continual leader in medical die cutting and medical converting.
We offer product development support, manufacture a small prototype quantity required for product validation, and produce high volume component quantities.
PLITEK® is certified to ISO 9001:2015 and ISO 13485:2016 standards while in the process of implementing and gaining compliance to Health Canada regulations.

Medical Converting
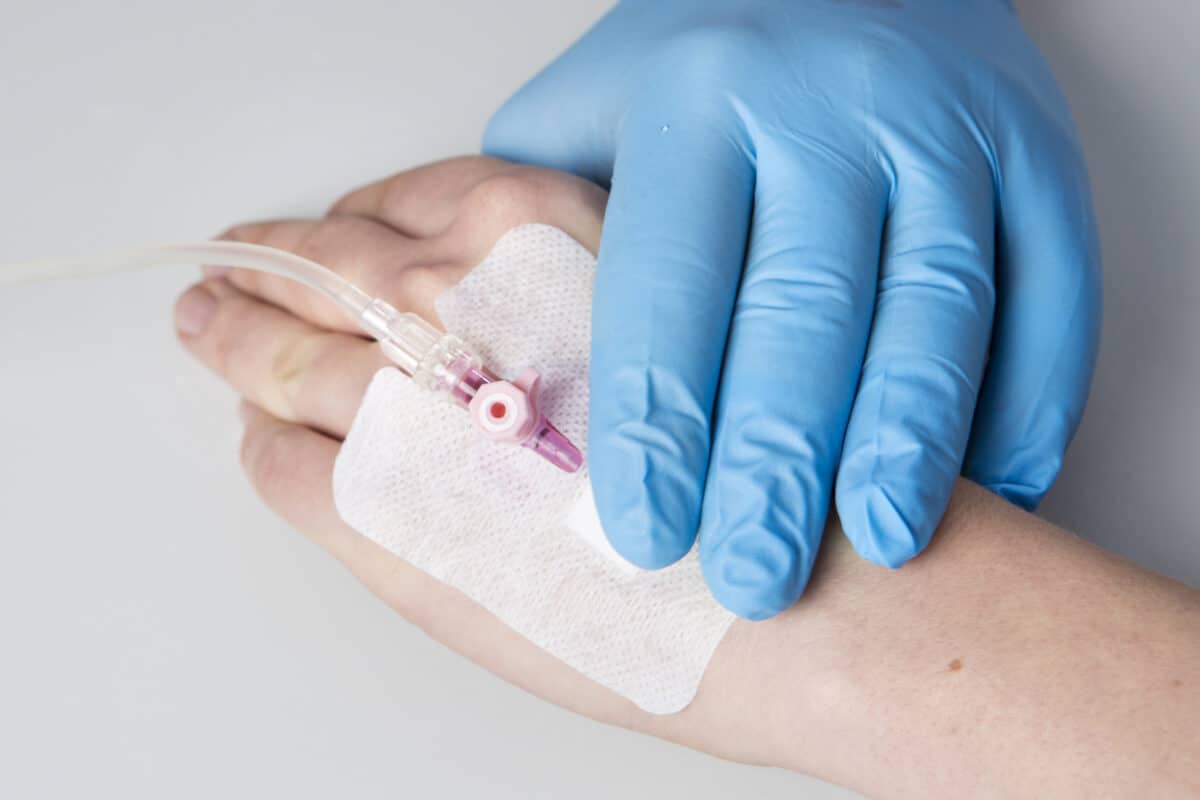
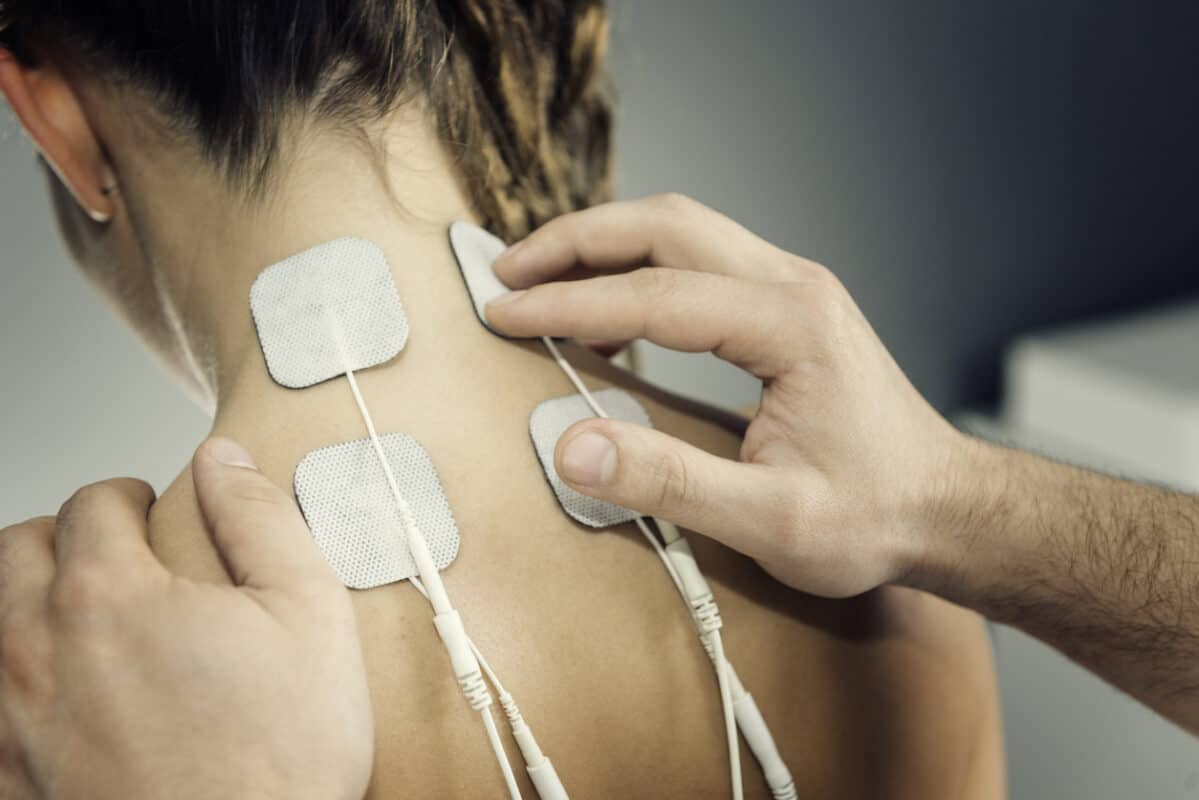
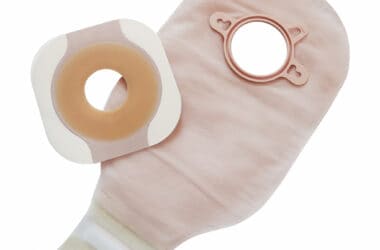
Medical Device Applications
- Diagnostics & Microfluidics
- Ostomy
- Wound care
- Cardiovascular/surgical
- Medical packaging
- Filtration
- Ophthalmic
Components and Materials
- PCR device
- Catheter and cannula holders
- Skin contact tapes, foams, and seals
- Wound dressings
- Multi-layer pads
- Hydrogel/hydrocolloid pads & electrodes
- Custom film extrusion
- Custom lamination
- Tethers
- Packaging/backing/insert cards
- Adhesive laminates
- Meltblown filter media
- Thermal interface materials








EXPLORE OUR CONVERTING CAPABILITIES:
Explore our capabilities
PLITEK® is an ISO 9001:2015 and ISO 13485:2016 certified global converting leader.